Thrust areas of research in our lab
Molecular Bioimaging
For the past few decades, fluorescence imaging mainly locates in the visible to NIR-I region (650 - 950 nm). The short wavelength (< 1000 nm) results in poor photon penetration depth. Compared with the visible and NIR-I light, second near-infrared (NIR-II, 1000 - 1700 nm) light can penetrate more deeply into biological tissues as less light is scattered at this long-wavelength window. NIR-II molecular fluorophore is the cornerstone of NIR-II fluorescence imaging. Recent years have witnessed a fast-increasing shift to the use of NIR-II fluorophores for in vivo imaging.
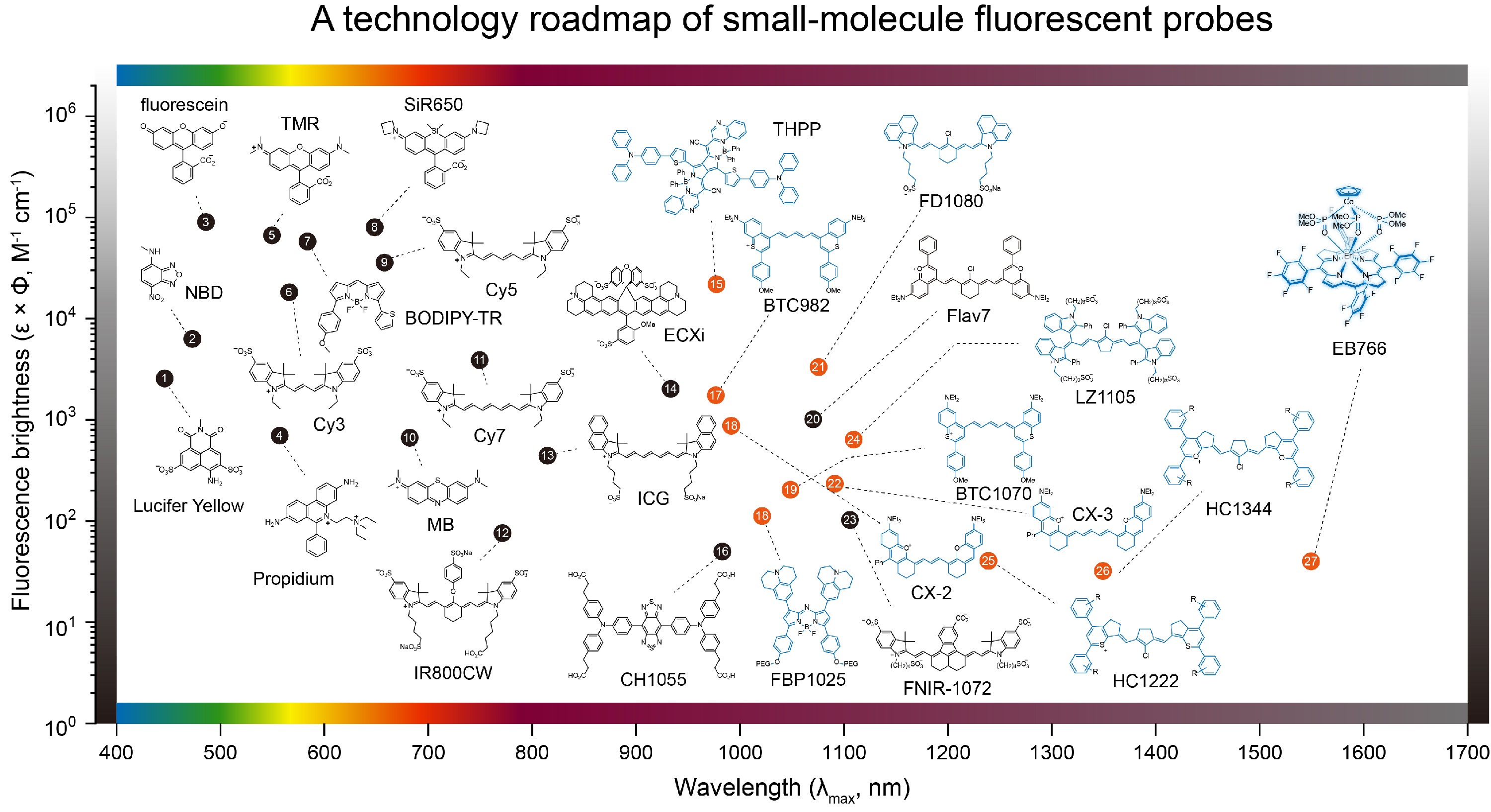
Nano Science and Engineering
Design, synthesis and surface bioconjugation of functional materials and structures on the nanometer scale. Examples include multicolor lanthanide nanocrystals, quantum dots for fluorescence imaging, magnetic nanoparticles for MRI, metallic nanoparticles for SPR, and mesoporous nanoparticles for targeted drug delivery.
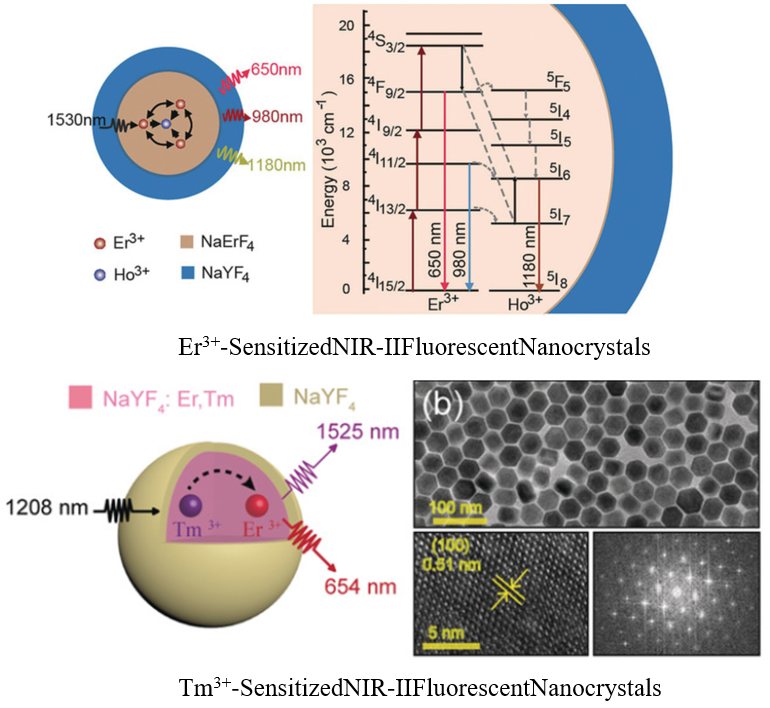
Angew. Chem. Int. Ed., 2018, 57(25), 7518-7522
Angew.Chem.Int.Ed.2019,58,10153–10157
Noninvasive In vivo bioimaging
Real-time monitoring of vessel dysfunction is of great significance in pre-clinical research. Optical bioimaging in the second near-infrared (NIR-II) window provides advantages including high resolution and fast feedback. Here, we report a series of NIR-II molecular probes with absorption and emission beyond 1000 nm. The fluorophore is used for continuous real-time monitoring of dynamic vascular processes, including ischemic reperfusion in hindlimbs, thrombolysis in carotid artery and opening and recovery of the blood brain barrier (BBB). These probes provide an approach for researchers to assess vessel dysfunction due to the long excitation and emission wavelength and long-term blood circulation properties.
Real-time NIR-II imaging of the ischemic reperfusion in hindlimbs
Real-time NIR-II imaging of the thrombolysis process in carotid artery
Intestinal motor
Nat Commun 11, 3102 (2020)
Nat. Mater. 20, 1571–1578 (2021)
Multiplexed noninvasive In vivo bioimaging
Spectrally distinct fluorophores are desired for multiplexed bioimaging. In particular, monitoring biological processes in living mammals need fluorophores that operate in the “tissue transparent” near-infrared (NIR) window, i.e. between 700-1700 nm. Here we report a fluorophore system based on molecular probes. We demonstrate its excitation/emission-multiplexed capability in the visualization of dynamic circulatory and metabolic processes in living mice, and through skull tracking of cancer cell metastases in mouse brain. This hybrid probe facilitates robust multiplexed NIR imaging with high contrast and spatial resolution for applications ranging from fluorescence-guided surgery, diagnostics and intravital microscopy.
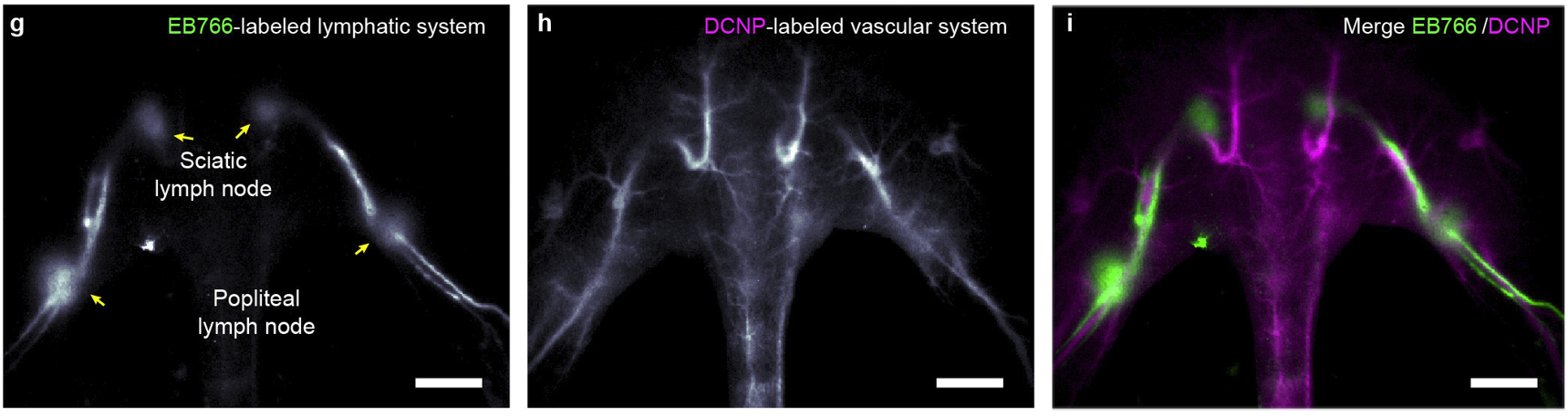
Nat. Mater. 20, 1571–1578 (2021)
Noninvasive In vivo microscopic bioimaging
Here, we designed an imaging system with a single excitation and simultaneous dual-channel detection in the NIR-IIb region, facilitating real-time dynamic multiplexed imaging utilizing the developed NIR-II probes. We achieved noninvasive multiplexed dynamic capturing of artery spontaneous vasomotion with high spatiotemporal synchronization. In addition, multiplexed imaging of cellular dynamics such as neutrophil adhesion, crawling and extravasation in the inflamed subcutaneous tissue and cerebral vessels of ischemic stroke mice was achieved noninvasively. Our technique for realizing noninvasive in vivo real-time dynamic multiplexing paves the way to reveal the realistic biological process without causing uncertain interferences in the microenvironment.
In vivo lifetime bioimaging
Deep tissue imaging in the second near-infrared (NIR-II) window holds great promise for physiological studies and biomedical applications. However, inhomogeneous signal attenuation due to biological matter hampers the application of multiple-wavelengths NIR-II probes to multiplexed imaging. Here we present lanthanide-doped NIR-II nanoparticles with engineered luminescence lifetimes for in vivo quantitative imaging using time-domain multiplexing. We demonstrate that robust lifetime coding is independent of tissue penetration depth, and we apply in vivo multiplexing to identify tumour subtypes in living mice.
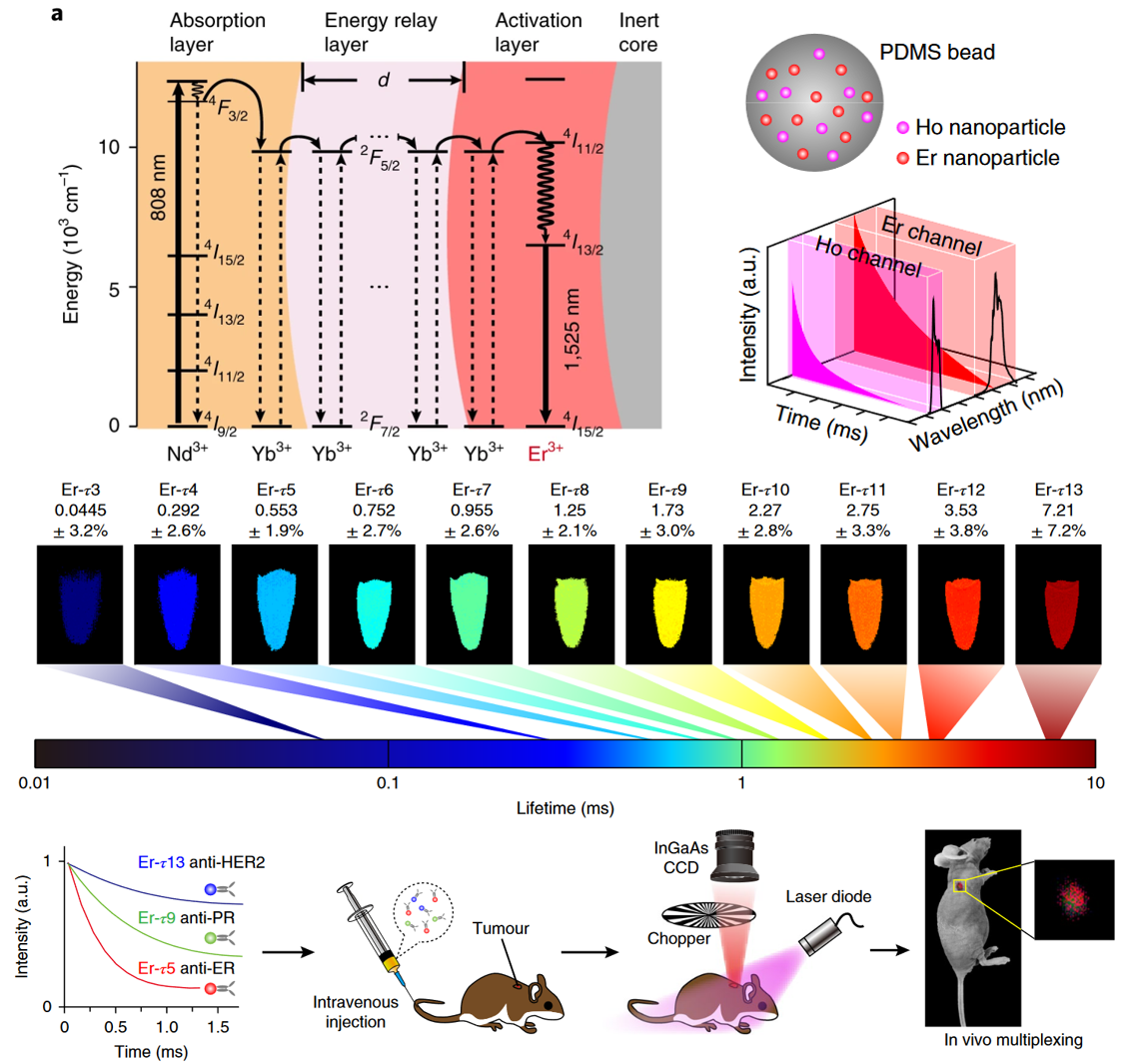
Nature Nanotech 13, 941–946 (2018)

